Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Li, L.*; Miyamoto, Goro*; Zhang, Y.*; Li, M.*; Morooka, Satoshi; Oikawa, Katsunari*; Tomota, Yo*; Furuhara, Tadashi*
Journal of Materials Science & Technology, 184, p.221 - 234, 2024/06
Times Cited Count:0 Percentile:0(Materials Science, Multidisciplinary) (cr) at 318 K in the presence of iron
(cr) at 318 K in the presence of ironYoshida, Yasushi*; Kitamura, Akira; Shibutani, Sanae*
Journal of Nuclear Science and Technology, 60(8), p.900 - 910, 2023/08
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)Ochs, M.*; Dolder, F.*; Tachi, Yukio
Applied Geochemistry, 136, p.105161_1 - 105161_11, 2022/01
Times Cited Count:4 Percentile:66.78(Geochemistry & Geophysics)Various types of radioactive wastes and environments contain organic substances that can stabilize the aqueous complexes with radionuclides and therefore lead to a decrease of sorption. The present study focuses on testing a methodology to quantify sorption reduction factors (SRFs) in the presence of organic ligands for cement systems. Three approaches for the estimation of SRFs; (1) analogy with solubility enhancement factors, (2) radionuclide speciation based on the thermodynamic calculations, and (3) experimental sorption data in ternary systems, were coupled and tested for the representative organic ligands (ISA and EDTA) and selected key radionuclides (actinides). Our approach allows to critically evaluate the dependence of SRFs for various systems on the chosen method of quantification, in accordance with the data availability for a given systems. The reliable SRFs can only be derived from the sorption measurements in ternary systems. SRF often need to be derived in the absence of such direct evidence, and estimations need to be made based on analogies and speciation information. However, such estimates may be subject to substantial uncertainties.
Rizaal, M.; Miwa, Shuhei; Suzuki, Eriko; Imoto, Jumpei; Osaka, Masahiko; Gou llo, M.*
llo, M.*
ACS Omega (Internet), 6(48), p.32695 - 32708, 2021/12
Times Cited Count:1 Percentile:6.77(Chemistry, Multidisciplinary)Kitamura, Akira
JAEA-Data/Code 2020-020, 164 Pages, 2021/03
Part of JAEA's Thermodynamic Database (JAEA-TDB) for solubility and speciation of radionuclides (JAEA-TDB-RN) for performance assessment of geological disposal of high-level radioactive and TRU wastes has been updated with subsuming the database for geochemical calculations (JAEA-TDB-GC). This report has focused to update JAEA-TDB-RN after selecting change in standard Gibbs free energy of formation (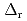
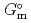 ), change in standard enthalpy change of formation (
), change in standard enthalpy change of formation (
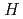

 ), standard molar entropy (
), standard molar entropy (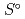
 ) and, heat capacity (
) and, heat capacity (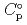 ), change in standard Gibbs free energy of reaction (
), change in standard Gibbs free energy of reaction (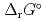
 ), change in standard enthalpy change of reaction (
), change in standard enthalpy change of reaction (
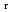
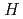

 ) and standard entropy change of reaction (
) and standard entropy change of reaction (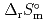 ) as well as logarithm of equilibrium constant (log
) as well as logarithm of equilibrium constant (log
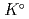 ) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
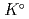 have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Kitamura, Akira; Yoshida, Yasushi*
Journal of Radioanalytical and Nuclear Chemistry, 327(2), p.839 - 845, 2021/02
Times Cited Count:3 Percentile:45.99(Chemistry, Analytical)Thermodynamic data for radium for radioactive waste management have been predicted using an electrostatic model and correlation with the ionic radii of the alkaline earth metals. Estimation of the standard Gibbs free energy of formation and standard molar entropy of aqueous radium species and compounds has been based on such approaches as extrapolation of the thermodynamic properties of strontium and barium, and use of a model of ion pair formation. The predicted thermodynamic data for radium have been compared with previously reported values.
Kitamura, Akira; Yoshida, Yasushi*; Goto, Takahiro*; Shibutani, Sanae*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 27(2), p.58 - 71, 2020/12
Evaluation and estimation of solubility values are required for a performance assessment of geological disposal of high-level radioactive and TRU wastes. Selection of solubility-limiting solid phases (SSPs) that control the solubility of radionuclides is necessary for the evaluation and estimation of solubility values. The authors have developed a methodology for selection of the SSP through a calculation of saturation indices (SIs) using thermodynamic database to show a transparent procedure for the selection. Literature survey should be performed to confirm decision of the SSP from candidate SSPs which generally have larger SIs from realistic point of view for precipitation and solubility control. The authors have selected the SSPs for the elements of interest for the latest Japanese performance assessment in bentonite and cement porewaters after grouping various water compositions.
Uno, Masayoshi*; Nishi, Tsuyoshi*; Takano, Masahide
Comprehensive Nuclear Materials, 2nd Edition, Vol.7, p.202 - 231, 2020/08
On the thermodynamic and thermophysical properties of the actinide nitrides in Comprehensive Nuclear Materials published by Elsevier as the first edition in 2012, we have revised them by adding some brand-new data. The main topics added are the solid solubility of the actinide nitrides into the zirconium nitride matrix for transmutation fuel, the lattice expansion of actinide nitrides induced by self-irradiation damage, the influence of defects accumulation on thermal conductivity, and the thermal expansion in curium nitride lattice.
Ikeuchi, Hirotomo; Yano, Kimihiko; Washiya, Tadahiro
Journal of Nuclear Science and Technology, 57(6), p.704 - 718, 2020/06
Times Cited Count:6 Percentile:60.71(Nuclear Science & Technology)To suggest efficient process of the fuel debris treatment after the retrieval from the Fukushima Daiichi Nuclear Power Plant (1F), thorough investigation is indispensable on potential source of U in the fuel debris. Estimation on the fuel debris accumulated in the reactor pressure vessel is specifically important due to its limited accessibility. The present study aims to estimate the chemical forms of U in the in-vessel fuel debris, especially in the minor phases such as metallic phases, by performing the thermodynamic calculation considering the material relocation and changing environment during the accident progression in the 1F Unit 2. Input conditions for the thermodynamic calculation such as composition, temperature, and oxygen amount were assumed mainly based on the results of severe accident analysis. The chemical form of U varied depending on the local amount of Fe and O. In regions of low steel content, the U-containing metallic phase was dominated by  -(Zr,U)(O), while regions of high steel content were dominated by Fe
-(Zr,U)(O), while regions of high steel content were dominated by Fe (Zr,U) (Laves phase). A few percent of U was transferred to the metallic phases under reducing conditions, raising challenging issues on the chemical removal of nuclear material from fuel debris.
(Zr,U) (Laves phase). A few percent of U was transferred to the metallic phases under reducing conditions, raising challenging issues on the chemical removal of nuclear material from fuel debris.
Miwa, Shuhei; Nakajima, Kunihisa; Miyahara, Naoya; Nishioka, Shunichiro; Suzuki, Eriko; Horiguchi, Naoki; Liu, J.; Miradji, F.; Imoto, Jumpei; Afiqa, B. M.; et al.
Mechanical Engineering Journal (Internet), 7(3), p.19-00537_1 - 19-00537_11, 2020/06
We constructed the fission product (FP) chemistry database named ECUME for LWR severe accident. This version of ECUME is equipped with dataset of the chemical reactions and their kinetics constants for the reactions of cesium(Cs)-iodine(I)-boron(B)-molybdenum(Mo)-oxygen(O)-hydrogen(H) system in gas phase, the elemental model for the high temperature chemical reaction of Cs with stainless steel applied as the structural material in a reactor, and thermodynamic data for CsBO vapor species and solids of Cs
vapor species and solids of Cs Si
Si O
O and CsFeSiO
and CsFeSiO for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
Miradji, F.; Suzuki, Chikashi; Nakajima, Kunihisa; Osaka, Masahiko
Journal of Physics and Chemistry of Solids, 136, p.109168_1 - 109168_9, 2020/01
Times Cited Count:2 Percentile:12.9(Chemistry, Multidisciplinary)Kitamura, Akira
Nihon Genshiryoku Gakkai-Shi ATOMO , 62(1), p.23 - 28, 2020/01
, 62(1), p.23 - 28, 2020/01
Thermodynamic databases (TDBs) for performance assessment of geological disposal of high-level waste and TRU waste have been developed to predict solubility and speciation of radionuclides in groundwater in some countries including Japan. The present manuscript briefly describes current status of development of the TDB organized by the Nuclear Energy Agency within the Organisation of Economic Co-operation and Development (OECD/NEA) and the TDBs in some countries including Japan.
 -UO
-UO
 -CO
-CO
 system and integration with JAEA's thermodynamic database for geochemical calculations
system and integration with JAEA's thermodynamic database for geochemical calculationsKitamura, Akira
JAEA-Data/Code 2018-018, 103 Pages, 2019/03
The latest available thermodynamic data were critically reviewed and the selected values were included into the JAEA-TDB for performance assessment of geological disposal of high-level radioactive and TRU wastes. This critical review specifically addressed thermodynamic data for (1) a zirconium-hydroxide system through comparison of thermodynamic data selected by the Nuclear Energy Agency within the Organisation for Economic Co-operation and Development (OECD/NEA), (2) complexation of metal ions with isosaccharinic acid based on the latest review papers. Furthermore, the author performed (3) tentative selection of thermodynamic data on ternary complexes among alkaline-earth metal, uranyl and carbonate ions, and (4) integration with the latest version of JAEA's thermodynamic database for geochemical calculations. The internal consistency of the selected data was checked by the author. Text files of the updated and integrated thermodynamic database have been prepared for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Miradji, F.; Suzuki, Chikashi; Nishioka, Shunichiro; Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko; Barrachin, M.*; Do, T. M. D.*; Murakami, Kenta*; Suzuki, Masahide*
Proceedings of 9th Conference on Severe Accident Research (ERMSAR 2019) (Internet), 21 Pages, 2019/03
Rai, D.*; Yui, Mikazu; Kitamura, Akira
Progress in Nuclear Science and Technology (Internet), 5, p.19 - 26, 2018/11
The objectives of this presentation are (1) to describe the solubility method, (2) to list desirable criteria of the solubility method so that the reader can recognize which studies have been done in a way that yields quality information, (3) to present an example of how to use the evaluation criteria, and (4) to provide a few examples of future research needs where the solubility method is ideally suited and the other methods are unsuitable for these investigations.
 (am) solubility at 25
(am) solubility at 25 C in the Ca
C in the Ca -Na
-Na -H
-H -Cl
-Cl -OH
-OH -H
-H O system; A Critical review
O system; A Critical reviewRai, D.*; Kitamura, Akira; Altmaier, M.*; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Journal of Solution Chemistry, 47(5), p.855 - 891, 2018/05
Times Cited Count:8 Percentile:8.45(Chemistry, Physical)We have critically reviewed experimental data for Zr hydrolysis constant values for formation of several mononuclear and polynuclear species and a solubility product value for ZrO (am). We have determined new/revised values for the formation constants of Zr(OH)
(am). We have determined new/revised values for the formation constants of Zr(OH)
 , Zr(OH)
, Zr(OH) (aq), Zr(OH)
(aq), Zr(OH)
 , Zr(OH)
, Zr(OH)
 and Ca
and Ca Zr(OH)
Zr(OH)
 , and the solubility product for ZrO
, and the solubility product for ZrO (am) after the critical review.
(am) after the critical review.
Ikeuchi, Hirotomo; Piluso, P.*; Fouquart, P.*; Excoffier, E.*; David, C.*; Brackx, E.*
Proceedings of 8th European Review Meeting on Severe Accident Research (ERMSAR 2017) (Internet), 12 Pages, 2017/05
no abstracts in English
Kitagaki, Toru; Yano, Kimihiko; Ogino, Hideki; Washiya, Tadahiro
Journal of Nuclear Materials, 486, p.206 - 215, 2017/04
Times Cited Count:29 Percentile:94.73(Materials Science, Multidisciplinary)Nakajima, Kunihisa
Mass Spectrometry (Internet), 5(2), p.S0055_1 - S0055_6, 2016/12
Though equilibrium vapor pressures are utilized to determine thermodynamic properties of not only gaseous species but also condensed phases, the obtained data often disagree by a factor of 100 and more. A new data analysis method is proposed using the so-called second and third law procedures to improve accuracy of vapor pressure measurements. It was found from examination of vapor pressures of cesium metaborate and silver that the analysis of the difference between the second and third law values can result in determination of an optimal data set. Since the new thermodynamic method does not require special techniques and or experiences in dealing with measured data, it is reliable and versatile to improve the accuracy of vapor pressure evaluation.
Amamoto, Ippei; Kobayashi, Hidekazu; Kitamura, Naoto*; Takebe, Hiromichi*; Mitamura, Naoki*; Tsuzuki, Tatsuya*; Fukayama, Daigen*; Nagano, Yuichi*; Jantzen, T.*; Hack, K.*
Journal of Nuclear Science and Technology, 53(10), p.1467 - 1475, 2016/10
Times Cited Count:3 Percentile:28.28(Nuclear Science & Technology)The iron phosphate glass (IPG) medium is known to be a high-efficiency glass medium, therefore we try to evaluate its applicability to immobilize sludge bearing radioactive nuclides arising from treatment of contaminated water at the stricken Fukushima Daiichi Nuclear Power Plant. For this study, many physical and chemical properties of target materials are necessary to evaluate the behaviours of IPG medium and its waste forms. Inevitably, it will entail the need for many and varied types of experiments to be carried out under high temperature. It is therefore rational to apply appropriate theoretical analysis first so as to reduce the number of experimental run. For this reason, some necessary thermodynamic values for theoretical analysis were estimated by CALPHAD approach followed by making up the calculated phase diagrams. By comparison with experimental results, they were found to be reliable for evaluating the behaviours of IPG medium and its waste forms.